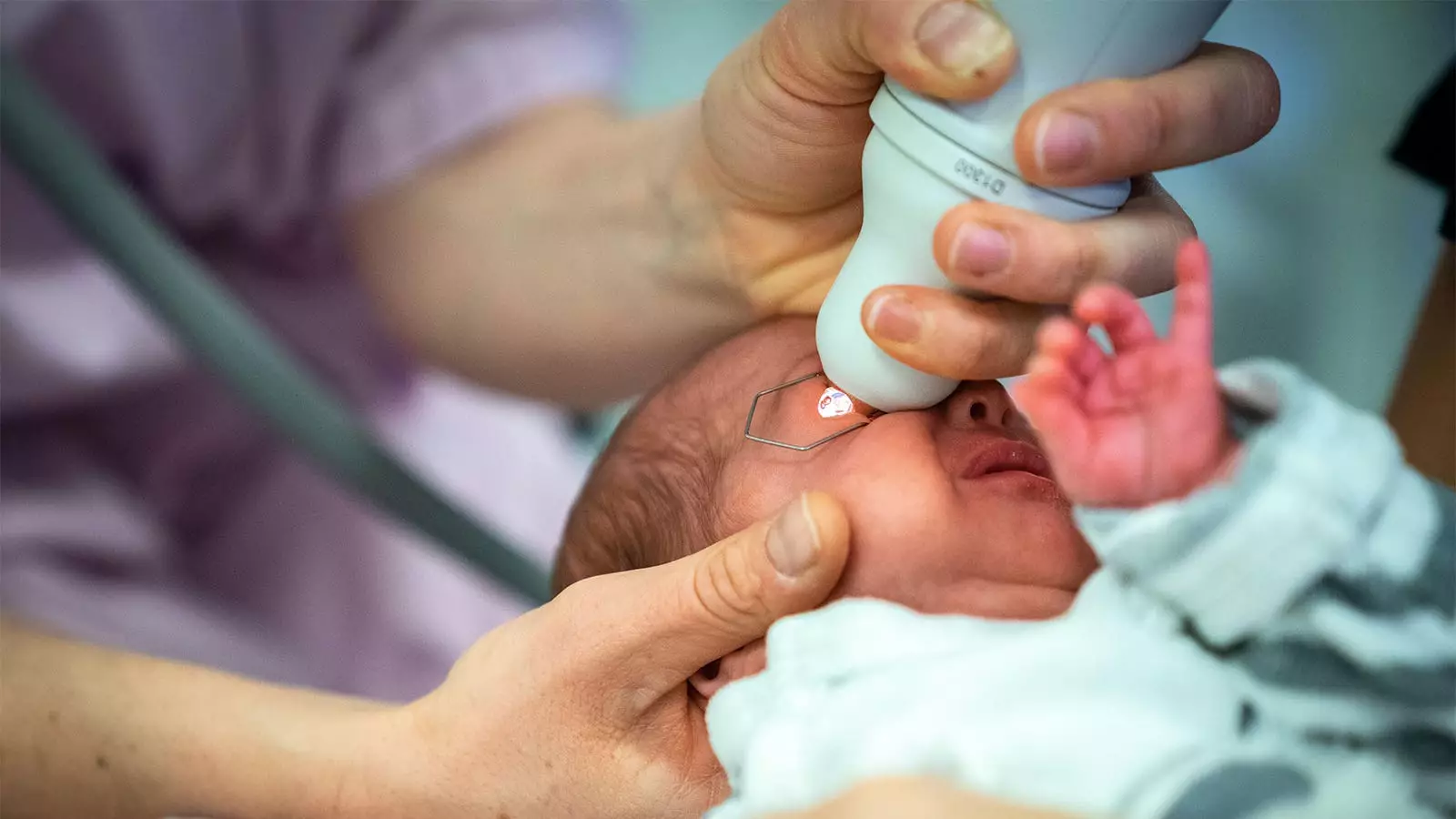
Retinopathy of Prematurity (ROP) is a significant concern in neonatal care, particularly for preterm infants. This eye disease can lead to blindness if not identified and treated early. Standard practices for ROP screening involve the use of mydriatic drops to dilate the pupils, allowing ophthalmologists to effectively examine the retina. However, the traditional mydriatic solutions have raised concerns regarding their safety profile, particularly the risk of cardiorespiratory and gastrointestinal adverse events in this fragile population. Recent research has aimed to explore alternatives that might mitigate these risks while maintaining diagnostic efficacy.
In a groundbreaking study conducted by Asimina Mataftsi, MD, PhD, and colleagues, the utility of mydriatic microdrops was thoroughly assessed against standard mydriatic drops. The study, which enrolled 83 preterm infants from a tertiary care center in Northern Greece, found that microdrops not only matched the effectiveness of conventional drops but also outperformed them regarding dilation achieved 45 minutes after administration. The mean difference in pupil diameter was noted to be statistically significant, providing robust evidence favoring microdrops over traditional formulations.
The microdrops, consisting of a diluted mix of phenylephrine and tropicamide, were administered in substantially smaller volumes (6.5 µL versus the standard 28 to 34 µL). This marked reduction in dosage appears to correspond with a lower incidence of adverse systemic effects, making microdrops a promising option for vulnerable infants.
One of the most critical findings of Mataftsi’s research was the decreased incidence of adverse effects linked to the use of microdrops. Infants treated with standard drops experienced lower oxygen saturation levels and a higher frequency of hypertensive episodes. These concerning outcomes underscore the fragility of preterm infants and their heightened susceptibility to medication-related complications. The data highlighted a median percentage of hypertensive episodes at 0.10% for microdrops compared to 0.14% for standard drops, showcasing a clear advantage in monitoring and managing the systemic stability of these infants during ROP examinations.
Previous literature has demonstrated a correlation between the administration of mydriatic agents and increased apnea events, adding credence to the need for refined approaches to ROP screening. By prioritizing the safety of these vulnerable patients, the study suggests that less invasive options such as microdrops are crucial in neonatal eye care.
The MyMiROPS study carefully selected participants based on strict criteria to ensure clarity and relevance in results. Eligible infants had a gestational age of less than 32 weeks or weighed less than 1,501 grams at birth. Given the mean gestational age of 29.7 weeks and a mean birth weight of 1,277 grams among participants, the study encapsulation reflects a population at high risk for ROP. Moreover, the randomized allocation of microdrops versus standard drops, alongside a decisive washout period, preserves the integrity of the study outcomes.
However, researchers did acknowledge certain limitations in their approach, particularly concerning the safety outcomes reported in 30% of the study cohort. This gap highlights the need for further investigations to comprehensively assess the long-term systemic exposure to these pharmacological agents.
The findings from this clinical trial represent a pivotal shift in the management of ROP screening among preterm infants. By demonstrating that mydriatic microdrops are not only effective but also safer than traditional drops, the study advocates for their broader adoption in neonatal care settings. Importantly, this research opens the door for future studies aimed at verifying these results, optimizing dosing regimens, and expanding clinical applications to varying patient populations. Continued efforts in this direction would be instrumental in enhancing neonatal care standards while protecting the delicate health of infants at risk for ROP.
Ultimately, transitioning to safer mydriatic options is not just a procedural change; it is a necessary evolution in protecting our most vulnerable patients’ health. The journey toward improving neonatal outcomes is ongoing, and the insights gleaned from these investigations are invaluable as healthcare providers strive to navigate the complexities of care in this sensitive population.