Ovarian cancer, particularly the aggressive form known as high-grade serous ovarian carcinoma (HGSOC), remains a significant challenge in gynecological oncology. Characterized by its rapid progression and grim prognosis—most patients succumb within five years of their initial diagnosis—early detection is paramount to improving survival rates. Recent research using murine models offers promising insights into the potential origins of this devastating disease. If these findings translate to human biology, they could herald a new era of early detection methods aimed at curbing the mortality associated with this cancer type.
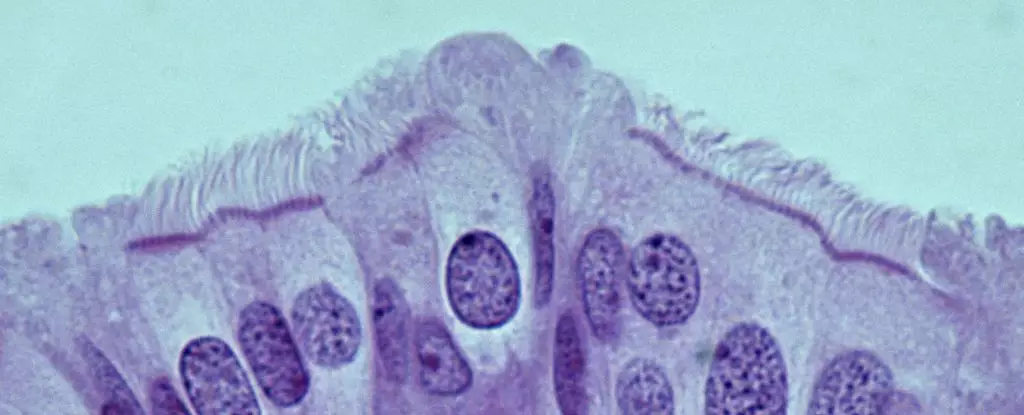
Traditionally, many believed that ovarian cancer primarily originated within the ovaries themselves. However, accumulating evidence over the past decade suggests that the seeds of this malignancy are often sown in the fallopian tubes. Researchers have noted that precursors to HGSOC, including atypical lesions, can occur at the tube’s ends, challenging long-held assumptions about the origin of the disease. The work of scientists has begun to illuminate this previously underappreciated area, showing that early changes in the fallopian tubes could lead to the development of ovarian tumors, highlighting the importance of these structures in understanding the disease’s pathology.
A pivotal breakthrough from recent studies led by Alexander Nikitin at Cornell University has identified specific cell types within the oviducts of mice that may be implicated in the onset of HGSOC. Unlike prior theories that pointed to stem cells as the culprits, Nikitin’s research reveals that pre-ciliated cells—those in the early stages of differentiation—are the most susceptible to malignant transformation. These cells are critical for the efficient transport of oocytes through the reproductive tract, but their vulnerability to genetic mutations associated with HGSOC calls for a reevaluation of how we view the cellular landscape of the fallopian tubes.
Intriguingly, the presence of certain genetic mutations appears to disrupt the normal function of pre-ciliated cells, leading to a fertile ground for cancer development. The connection between cilia formation abnormalities and cancer is not limited to ovarian cancer; similar disruptions have been studied in other malignancies, such as pancreatic cancer. This raises an essential question about the interconnectedness of these diseases and suggests that the pathways leading to tumorigenesis could share commonalities, which may eventually guide targeted therapeutic interventions.
The critical insight from identifying cancer-prone cells in murine oviducts paves the way for potential breakthroughs in human medicine. If scientists can pinpoint similar mechanisms operating within the human fallopian tubes, it would open doors for developing novel diagnostic markers and therapeutic strategies for HGSOC. Currently, with about 80% of HGSOC cases diagnosed at advanced stages due to the absence of early symptoms, this research could drastically alter the landscape of ovarian cancer diagnosis and treatment.
While the road from translational research to clinical application is fraught with challenges, the identification of pre-ciliated cells as potential instigators of HGSOC represents a significant leap forward. The focus should now shift to further exploring the biological mechanisms behind these findings and determining whether other genetic mutations associated with HGSOC affect different cellular pathways. The relentless pursuit of understanding the interplay between these cells and cancer could lead to innovative screening tools, ultimately saving countless lives by facilitating earlier intervention and treatment. The promise of translating these murine discoveries into effective human therapies embodies hope in the ongoing battle against ovarian cancer.