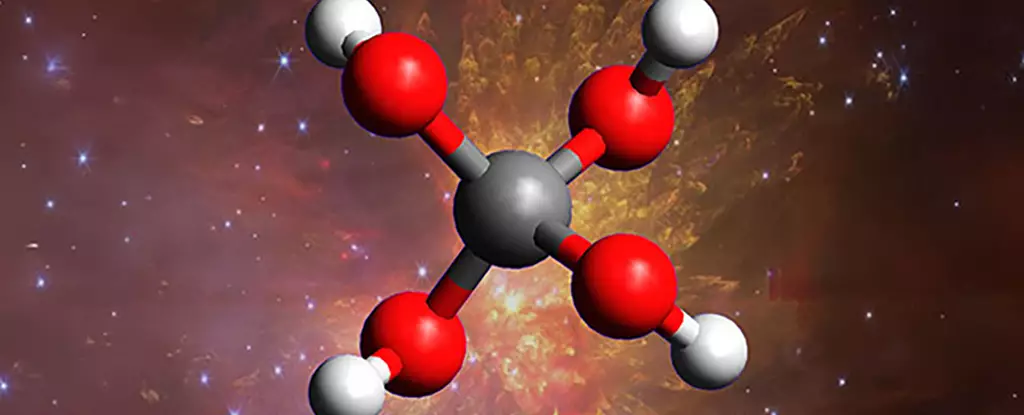
In recent scientific breakthroughs, researchers have claimed to synthesize an unprecedented molecule called methanetetrol—a so-called “super alcohol” composed of four hydroxyl groups standing alone on a single carbon atom. At first glance, this discovery appears to be a remarkable leap forward in understanding the universe’s chemical tapestry, fueling optimism about uncovering alien life and unraveling cosmic mysteries. However, beneath the surface, this excitement reveals a fundamental misjudgment of scientific realism and overreach. The leap from laboratory simulation to cosmic reality is vast, and the enthusiasm surrounding such “impossible” molecules often obscures the true complexity of space chemistry, which remains largely uncharted and far more restricted than researchers assume.
The Overhyped Narrative of Space Chemistry
While unveiling new molecules captivates the imagination, the reality is that we are still venturing into a largely unexplored chemical universe. The detection of such fragile molecules as methanetetrol in laboratory conditions—created under conditions mimicking space—does not directly translate to their existence beyond Earth. The molecule’s extreme instability, which causes it to rapidly dissociate when exposed to light or radiation, underscores how fleeting such entities are, making their natural occurrence in space improbable. The narrative spun by scientists seems to lean heavily on speculation, igniting public fascination with a story that oversimplifies the harsh realities of deep space conditions. It’s a reminder that scientific breakthroughs, especially those leading to sensational headlines, must be critically assessed for their actual relevance and implications.
The Risks of Overinterpreting Space Simulations
The drive to uncover extraterrestrial molecules often leads scientists to push the boundaries of current technology and interpretation. Yet, simulating space in a lab—by freezing carbon dioxide, water, and bombarding with radiation—remains an approximation, not a reproduction. This liminal space between experiment and cosmos is rife with pitfalls. The assumption that molecules like methanetetrol exist naturally in deep space ignores the fundamental constraints imposed by energy, stability, and environmental factors. For example, the molecule’s propensity for dissociation under irradiation suggests it cannot withstand the long, cold, radiation-sparse environment of space over cosmic timescales. Consequently, the excitement generated by this discovery might be misdirected, recasting what is likely a laboratory curiosity as a profound cosmic truth.
The Question of Scientific Overreach and Public Hype
The media and scientific community often elevate such findings as revolutionary, but the truth is that we are still only scratching the surface of space chemistry. The claim that this molecule could be pivotal for understanding the origins of life stretches credulity. Scientific progress is incremental; overhyped narratives not only mislead the public but also risk diverting resources from more pressing, empirically grounded research. The allure of discovering “impossible” molecules can blind us to the fact that the universe’s chemistry is governed by laws more restrictive than our laboratory models suggest. It’s a cautionary tale about the importance of moderation in scientific communication—acknowledging what we do not yet know rather than overstating what we do.
False Promises and the Need for Cautious Optimism
While the pursuit of understanding cosmic molecules should continue, it must be tempered with skepticism. The notion that molecules like methanetetrol are hiding in space, waiting for us to find them, is overly optimistic. Our technological limitations, coupled with the inherently unstable nature of these molecules, imply that their existence in large, detectable quantities remains unlikely. Instead of investing heavily in the quest to observe such fleeting species, the focus should shift toward developing methods for identifying more stable chemical signatures and understanding the broader context of space chemistry. Real progress depends on patience, precision, and a healthy dose of skepticism—not on the exaggerated promises of a scientific breakthrough that may, in reality, be a mirage.
The Critical Path Forward
Moving forward, we need a balanced perspective—one that recognizes the importance of such research while critically evaluating its implications. The history of scientific discovery shows that the most profound insights often come from persistent inquiry into the mundane, the stable, and the observable, rather than chasing after ephemeral miracles. Instead of being seduced by the allure of “impossible” molecules, we should focus on refining our detection techniques, expanding our understanding of space’s chemical constraints, and maintaining an honest dialogue about what these discoveries truly signify. Only with such a cautious yet curious approach can we hope to avoid the pitfalls of hype and make genuine progress in unraveling the universe’s complex chemical fabric.